Coinfection with Chlamydia abortus y Coxiella burnetii in cows, goats and ewes that had abortion
Keywords:
Coxiella burnetii, Chlamydia abortus, coinfection, rumiantsAbstract
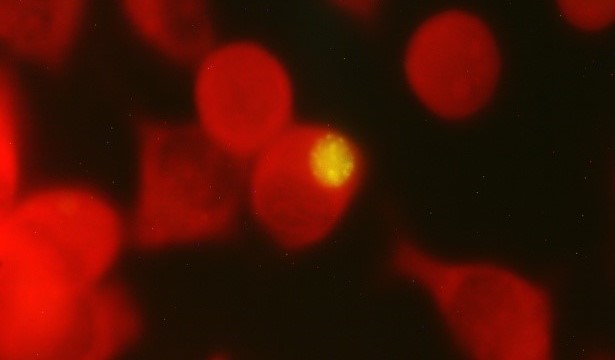
The aim of this study was to determine the coinfection with Chlamydia abortus and Coxiella burnetii, in cows, goats, and sheep that had abortion. The study analyzed 77 vaginal swabs; animals included 20 cows, 40 goats and 17 sheep. Bacterial isolation was performed using L929 mouse fibroblasts. The identification of inclusion bodies in the infected cell cultures was carried out using the direct immunofluorescence (IFD) technique using an IMAGEN Chlamydia test kit (IMAGEN™ Thermo Scientific, USA) and molecular identification was achieved by ompA gene (species-specific) real-time polymerase chain reaction (PCR). PCR was used for the detection of C. burnetii DNA in vaginal swabs. The cell culture isolation and real-time PCR species-specific PCR revealed the presence of C. abortus in 100% of samples. In this study, 63 samples (81.8%) of the 77 collected samples were positive for C. burnetii. showing coinfection with C. abortus; being 55% in cows; 95% in goats, and 82% in sheep. The results indicate that the coinfection caused by C. abortus and C. burnetii in cows, goats and sheep with a history of abortion, represents the first report in Mexico.
http://dx.doi.org/10.21929/abavet2024.10
e2023-107
https://www.youtube.com/watch?v=u9e-ZrnTxKU
References
ALI H, Al-Bayati LH. 2022. Serological and histopathological investigation of Chlamydia abortus in aborted ewes in Wasit, Iraq. Archives of razi institute. 77(3):1105–1111. https://doi.org/10.22092/ARI.2022.357270.2009
ARAUJO-MELÉNDEZ J, Sifuentes OJ, Bobadilla JM, Aguilar CA, Torres AO, Ramirez GJ, Ponce A, Ruiz PG, Guerrero AM. 2012. What do we know about Q fever in Mexico? Revista de investigación clínica. 64(61):541-545.
https://pubmed.ncbi.nlm.nih.gov/23513611/
BASANISI MG, La Bella G, Nobili G, Raele DA, Cafiero MA, Coppola R, Damato AM, Fraccalvieri R, Sottili R, La Salandra G. 2022. Detection of Coxiella burnetii DNA in sheep and goat milk and dairy products by droplet digital PCR in south Italy. International journal of food microbiology. 366, e109583.
https://doi.org/10.1016/j.ijfoodmicro.2022.109583
DE BRUIN A, De Groot A, De Heer L, Bok J, Wielinga PR, Hamans M, Van Rotterdam BJ, Janse I. 2011. Detection of Coxiella burnetii in complex matrices by using multiplex quantitative PCR during a major Q fever outbreak in The Netherlands. Applied and environmental microbiology. 77(18):6516-6523.
https://pubmed.ncbi.nlm.nih.gov/21784920/
Diario oficial de la federación (DFO). 2018. Acuerdo mediante el cual se dan a conocer en los Estados Unidos Mexicanos las enfermedades y plagas exóticas y endémicas de notificación obligatoria de los animales terrestres y acuáticos. México. https://dof.gob.mx/nota_detalle.php?codigo=5545304&fecha=29/11/2018
ESCALANTE OC, Díaz AE, Segundo ZC, Suárez GF. 1997. Isolation of Chlamydia psittaci involved in abortion of goats in Mexico: first report. Revista latinoamericana de microbiología. 39(3-4):117–121. PMID: 10932720.
https://pubmed.ncbi.nlm.nih.gov/10932720/
FAYEZ M, Elmoslemany A, Alorabi M, Alkafafy M, Qasim I, Al-Marri T, Elsohaby I. 2021. Seroprevalence and risk factors associated with chlamydia abortus infection in sheep and goats in eastern Saudi Arabia. Pathogens (Basel, Switzerland). 10(4):489. https://doi.org/10.3390/pathogens10040489
FLORES PCF, Díaz AE, Palomares REG., Isidro RLM., Herrera LE, Hernández CR. 2022. Molecular identification of Coxiella burnetii in vaginal swabs from aborted goats in Mexico. Small ruminant research. 210, e106664.
https://doi.org/10.1016/j.smallrumres.2022.106664
KREIZINGER Z, Szeredi L, Árpád Bacsadi A, Nemes C, Sugár L, Varga T, Sulyok KM, Szigeti A, Ács k, Tóbiás e, Borel N, Gyuranecz M. 2015. Occurrence of Coxiella burnetii and Chlamydiales species in abortions of domestic ruminants and in wild ruminants in Hungary, Central Europe. Journal of veterinary diagnostic investigation. 27(2):206-210. https://doi.org/10.1177/1040638714563566
LEYVA CJC, Palomares REG, Gutiérrez HJL, Herrera LE, Aguilar RF, Morales PMI, Mejía SP, Díaz AE. 2021. serological evidence of antibodies against coxiella burnetii in sheep flocks of México. Journal of animal and veterinary advances. 20(5):114-117. https://medwelljournals.com/abstract/?doi=javaa.2021.114.117
LIMÓN GM. 2022. Genotipificación de aislamientos de Chlamydia spp. obtenidos a partir de abortos en rumiantes. Tesis de Doctorado. Universidad Nacional Autónoma de México. https://ru.dgb.unam.mx/handle/DGB_UNAM/TES01000821694
LIMÓN GM, Hernández CR, Martínez HF, Xicohtencatl CJ, Ramírez AH, Palomares REG, Díaz AE. Genetic diversity of Chlamydia pecorum detected in sheep flocks from Mexico. 2022. Brazilian journal of microbiology. 53(2):605-613.
https://doi.org/10.1007/s42770-022-00682-9
MARES-GUÍA M, Gutiérrez A, Rozental T, Ferreira MDS, Lemos ERS. 2018. Clinical and epidemiological use of nested PCR targeting the repetitive element IS1111 associated with the transposase gene from Coxiella burnetii. Brazilian journal of microbiology. 49(1):138-143. https://doi.org/10.1016/j.bjm.2017.04.009
MARTÍNEZ SMG, Palomares RG, Tórtora PJL, Ramírez AH, Ortega HN, Salinas LJ, Morales AJF, Cervantes MJJC, Díaz AE. 2022. Presence of Chlamydia abortus in colostrum, milk and vaginal discharge samples of sheep. Revista colombiana ciencias pecuarias. 35(3):165–173. https://doi.org/10.17533/udea.rccp.v35n2a04
MORA DJ, Díaz AE, Herrera LE, Suárez GF, Escalante OC, Jaimes VS, Arellano RB. 2015. Isolation of Chlamydia abortus in dairy goat herds and its relation to abortion in Guanajuato, Mexico. Veterinaria méxico oa. 2(1). https://doi.org/10.21753/vmoa.2.1.339
MUEMA J, Nyamai M, Wheelhouse N, Njuguna J, Jost C, Oyugi J, Bukania Z, Oboge H, Ogoti B, Makori A, Fernandez MDP, Omulo S, Thumbi SM. 2022. Endemicity of Coxiella burnetii infection among people and their livestock in pastoral communities in northern Kenya. Heliyon. 8(10), e11133. https://doi.org/10.1016/j.heliyon.2022.e11133
PANTCHEV A, Sting R, Bauerfeind R, Tyczka J, Sachse K. 2010. Detection of all Chlamydophila and Chlamydia spp. of veterinary interest using species-specific real-time PCR assays. Comparative immunology, microbiology and infectious diseases. 33(6):473–484. https://doi.org/10.1016/j.cimid.2009.08.002
PEARSON T, Hornstra HM, Hilsabeck R, Gates LT, Olivas SM, Birdsell DM, Hall CM, German S, Cook JM, Seymour ML, Priestley, RA, Kondas AV, Clark Friedman CL, Price EP, Schupp JM, Liu CM, Price LB, Massung RF, Kersh GJ, Keim P. 2014. High prevalence and two dominant host-specific genotypes of Coxiella burnetii in U.S. milk. BMC Microbiology. 14:41. https://doi.org/10.1186/1471-2180-14-41
RAMO MLA, Benito AA, Quílez J, Monteagudo LV, Baselga C, Tejedor MT. 2022. Coxiella burnetii and co-infections with other major pathogens causing abortion in small ruminant flocks in the iberian peninsula. Animals (Basel). 12(24), e3454.
https://doi.org/10.3390/ani12243454
RUNGE M, Binder A, Schotte U, Ganter M. 2012. Investigations concerning the prevalence of Coxiella burnetii and Chlamydia abortus in sheep in correlation with management systems and abortion rate in lower saxony in 2004. Berliner und Munchener tierarztliche Wochenschrift. 125(3-4):138–143.
https://pubmed.ncbi.nlm.nih.gov/22515032/
SANTAMARÍA JR. 2009. Fiebre Q en el Estado de Hidalgo, México. Reporte de caso. Perinatología y Reproducción Humana. 23(1):34-37. ISSN 0187-5337.
https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=21247
SÁNCHEZ RL. Arellano RB, Hernández CR, Palomares REG, Barradas PF, Díaz AE. 2021. Presence of Chlamydia abortus in goats with a history of abortions in Mexico. Abanico veterinario. 11(1), e2021-2. ISSN 2448-6132.