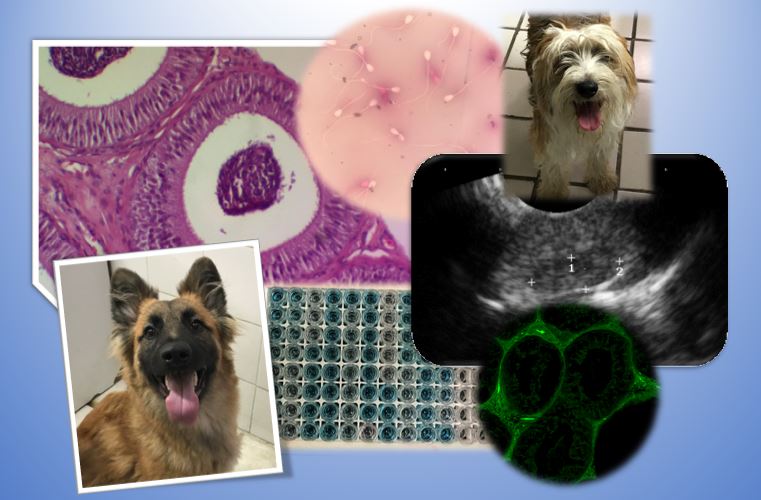
Efecto del coumestrol sobre el epidídimo de perros adultos
Palabras clave:
epidídimo, espermatozoides, estrógenos, fitoestrógenos, testículo, perro, control reproductivoResumen
La preocupación de la sobrepoblación canina se relaciona con zoonosis como la rabia, la cual es responsable del 99% de los casos de rabia humana, causante de la muerte de aproximadamente 59,000 personas al año. La esterilización quirúrgica es un método de control eficaz, costoso e invasivo siendo su impacto limitado. Los efectos de los fitoestrógenos en la actividad reproductiva se han estudiado comúnmente en hembras, y un número limitado en machos. Por lo anterior, el objetivo de este estudio fue conocer el efecto de la administración subcutánea de coumestrol en la actividad gonadal de perros adultos como alternativa para su control reproductivo. Se determinaron los parámetros de evaluación seminal básica, la estructura del epidídimo mediante ecografía, características histológicas, así como la presencia del coumestrol mediante fluorescencia y los niveles séricos de testosterona y estrógenos. La administración de coumestrol durante cinco semanas redujo la producción espermática, y evidenció cambios en la eco densidad y celularidad del epidídimo, asociados a las concentraciones séricas de estradiol y testosterona. Por lo que, se concluye que el coumestrol administrado vía subcutánea tiene un efecto estrogénico que puede utilizarse como un método no invasivo para ayudar a controlar la fertilidad de perros adultos.
http://dx.doi.org/10.21929/abavet2022.15
https://www.youtube.com/watch?v=6d4WqxZqAqo
e2022-17
Citas
ALDER SA, Purup S, Hansen-Møller J, Thuen E, Steinshamn H. 2015. Phytoestrogens and their metabolites in bulk-tank milk: effects of farm management and season. PLoS One.10(5): e0127187. https://doi.org/10.1371/journal.pone.0127187
ALI HASSAN H, Domain G, Luvoni GC, Chaaya R, Van Soom A, Wydooghe E. 2021. Canine and Feline Epididymal Semen—A Plentiful Source of Gametes. Animals.11:2961. https://doi.org/10.3390/ani11102961
ASA CS. Contraception in Dogs and Cats. 2018. Vet Clin North Am Small Anim Pract. 48(4):733-742. https://doi.org/10.1016/j.cvsm.2018.02.014
AZIZ SJ, Zeman-Pocrnich CE. 2022. Tissue Processing. Methods Mol Biol. 2422:47-63. https://doi.org/10.1007/978-1-0716-1948-3_4
BARBOSA DE SOUZA M, England GCW, Mota Filho ACAckermann CL, Vládia Soares Sousa CV, Guedelha de Carvalho G, Rodrigues Silva HV, Pinto JN, Spíndola Linhares JC, Oba E, Machado da Silva LD. 2015. Semen quality, testicular B-mode and Doppler ultrasound, and serum testosterone concentrations in dogs with established infertility. Theriogenology. 84: 805–810. https://doi.org/10.1016/j.theriogenology.2015.05.015
BELSARE A, Vanak AT. 2020. Modelling the challenges of managing free-ranging dog populations. Sci Rep. 10(1):18874. https://doi.org/10.1038/s41598-020-75828-6
BESZTERDA M, Frański R. 2018. Endocrine disruptor compounds in environment: As a danger for children health. Pediatric Endocrinology Diabetes and Metabolism. 24(2):88-95. https://doi.org/10.18544/PEDM-24.02.0107
CERUNDOLO R, Michel KE, Reisner IR, Phillips L, Goldschmidt M, Court MH, Shrestha B, Hao Q, Refsal K, Oliver JW, Biourge V, Shofer FS. 2009. Evaluation of the effects of dietary soy phytoestrogens on canine health, steroidogenesis, thyroid function, behavior and skin and coat quality in a prospective controlled randomized trial. American Journal of Veterinary Research. 70(3): 353–360. https://doi.org/10.2460/ajvr.70.3.353
CHŁOPIC A, Wysokińska A. 2020. Canine spermatozoa—What do we know about their morphology and physiology? An overview. Reproduction in Domestic Animals. 55:113–126. https://doi.org/10.1111/rda.13596
COOKE PS, Mesa AM, Sirohi VK, Levin ER. 2021. Role of nuclear and membrane estrogen signaling pathways in the male and female reproductive tract. Differentiation. 118:24-33. https://doi.org/10.1016/j.diff.2020.11.002
CORNWAL GA. 2009. New insights into epididymal biology and function. Human Reproduction Update. 15(2):213–227. https://doi.org/10.1093/humupd/dmn055
DOMÍNGEZ-LÓPEZ I, Yago-Aragón M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. 2020. Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients.12: 2456. https://doi.org/10.3390/nu12082456
EVANS MJ, Gibson A, Fielding H, Ohal P, Pandey P, Kumar A, Singh SK, Airikkala-Otter I, Abela-Ridder B, Gamble L, Handel I, Bronsvoort BMDC, Mellanby RJ, Mazeri S. 2022. Free-roaming dog population dynamics in Ranchi, India. Research in Veterinary Science. 143:115-123. https://doi.org/10.1016/j.rvsc.2021.12.022
HAMMER Ø, Harper DAT, Ryan PD. 2001. Past: Paleontological Statistics Software Package for Education and Data Analysis. Paleontología Electrónica. 4:1 https://palaeo-electronica.org/2001_1/past/issue1_01.htm
HAMPSON K, Abela-Ridder B, Bharti O, Knopf L, Léchenne M, Mindekem R, Tarantola A, Zinsstag J, Trotter C. 2019. Modelling to inform prophylaxis regimens to prevent human rabies. Vaccine. 37(1(Suppl 1)): A166-A173.
https://doi.org/10.1016/j.vaccine.2018.11.010
HAMPSON K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin FX, Metlin A, Miranda ME, Müller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MA, Zinsstag J, Dushoff J. 2015. Global Alliance for Rabies Control Partners for Rabies Prevention. Estimating the global burden of endemic canine rabies. PLoS Neglected Tropical Diseases. 9(4): e0003709.
https://doi.org/10.1371/journal.pntd.0003709
HESS RA, Cooke PS. 2018. Estrogen in the male: a historical perspective. Biology of Reproduction. 99(1):27–44. https://doi.org/10.1093/biolre/ioy043
JOHNSTON SD. 1991. Performing a complete canine semen evaluation in a small animal hospital. Veterinary Clinics of North America Small Animal Practice. 21(3):545-51. https://doi.org/10.1016/s0195-5616(91)50060-7
KAWAKAMI E, Hirano T, Hori T, Tsutsui T. 2004. Improvement in spermatogenic function after subcutaneous implantation of a capsule containing an aromatase inhibitor in four oligozoospermic dogs and one azoospermic dog with high plasma estradiol-17b concentrations. Theriogenology. 62: 165–178.
https://doi.org/10.1016/j.theriogenology.2003.09.021
KUROWICKA B, Dietrich GJ, Kotwica G. 2015. Effect of neonatal or adult heat acclimation on testicular and epididymal morphometry and sperm production in rats. Reproductive Biology. 15(1):1-8. https://doi.org/10.1016/j.repbio.2015.01.001
LEOCI R, Aiudi G, Silvestre F, Lissner EA, Marino F, Lacalandra GM. 2015. Therapeutic Ultrasound as a Potential Male Dog Contraceptive: Determination of the Most Effective Application Protocol. Reproduction in Domestic Animals. 50(5):712-8.
https://doi.org/10.1111/rda.12548
LEPHART ED. 2015. Modulation of Aromatase by Phytoestrogens. Enzyme Research. 594656. https://doi.org/10.1155/2015/594656
LUBINUS BADILLO FG, Buitrago Aguilar C. 2006. Lesiones testiculares benignas: hallazgos ecográficos. Med UNAB. 9 (2):120-127.
https://revistas.unab.edu.co/index.php/medunab/article/view/153
MANTZIARAS G. 2020. Imaging of the male reproductive tract: Not so easy as it looks like. Theriogenology. 150:490e497. https://doi.org/10.1016/j.theriogenology.2020.03.009
MASSEI G, Miller LA. 2013. Nonsurgical fertility control for managing free-roaming dog populations: a review of products and criteria for field applications. Theriogenology. 80(8):829-838. https://doi.org/10.1016/j.theriogenology.2013.07.016
MIRANDA-CASTRO SP, Lizarraga-Paulin E. 2012. Is Chitosan a New Panacea? Areas of Application. In: The Complex World of Polysaccharides. (Karunaratne DN Ed). Intech Publisher, Croatia. Pp. 1-44. https://doi.org/10.5772/51200
MORALES BM, Rodrigues da Rosa Filho, JR, Agostini LD, Infantosi VC. 2021. Ageing changes testes and epididymis blood flow without altering biometry and echodensity in dogs. Animal Reproduction Science. 228:106745.
https://doi.org/10.1016/j.anireprosci.2021.106745
MOTA-ROJAS D, Calderón-Maldonado N, Lezama-García K, Sepiurka L, Maria Garcia RC. 2021. Abandonment of dogs in Latin America: Strategies and ideas. Veterinary World. 14(9):2371-2379. https://doi.org/10.14202/vetworld.2021.2371-2379.
MOSTROM M, Evans TJ. 2018. Chapter 60 “Phytoestrogens”, Editor(s): Ramesh C. Gupta, Veterinary Toxicology (Third Edition), Academic Press, Pages 817-833, ISBN 9780128114100. https://doi.org/10.1016/B978-0-12-811410-0.00060-X
NCRRRAR. National Centre For The Replacement, Refinement, and Reduction of Animal in Research. The 3 R. http://www.nc3rs.org.uk/the-3rs
NIH. National Institutes of Health. Image Processing and Analysis in Java (ImageJ). http://rsb.info.nih.gov/ij/
NIE R, Zhou Q, Jassim E, Saunders PTK, Hess RA. 2002. Differential Expression of Estrogen Receptors a and b in the Reproductive Tracts of Adult Male Dogs and Cats. Biology of Reproduction. 66:1161-1168. https://doi.org/10.1095/biolreprod66.4.1161
PEÑA-CORONA S, León P, Mendieta E, Villanueva M, Salame A, Vargas D, Mora G, Serrano H, Villa-Godoy A. 2019. Effect of a single application of coumestrol and/or dimethyl sulfoxide, on sex hormone levels and vaginal cytology of anestrus bitches. Veterinaria México OA, 6(1), 52-66. Epub 20 de febrero de 2020. https://doi.org/10.22201/fmvz.24486760e.2019.1.656
PEREZ-RIVERO JJ, Pérez-Martínez M, Aguilar-Setién A. 2014. Histometric analysis of vampire bat (Desmodus rotundus) testicles treated with coumestrol by oral route. Journal of Applied Animal Research. 42:2:208-212.
https://doi.org/10.1080/09712119.2013.827578
PEREZ-RIVERO JJ, Martinez-Maya JJ, Pérez-Martinez M, Aguilar-Setien A, García-Suarez MD, Serrano H. 2009. Phytoestrogen treatment induces testis alterations in dogs. Potential use in population control. Veterinary Research Communications. 33:87–95. https://doi.org/10.1007/s11259-008-9077-3
RIETJENS IMCM, Louisse J, Beekmann K. 2017. The potential health effects of dietary phytoestrogens. British Journal of Pharmacology. 174(11):1263-1280. https://doi.org/10.1111/bph.13622
ROOT KUSTRITZ MV. 2018. Population Control in Small Animals. Veterinary Clinics of North America Small Animal Practice. 48(4):721-732. https://doi.org/10.1016/j.cvsm.2018.02.013
RUBEL D, Carbajo A. 2019. Dogs in public spaces of Buenos Aires, Argentina: Exploring patterns of the abundance of dogs, the canine faecal contamination, the behaviour of people with dogs, and its relationships with demographic/economic variables. Preventive Veterinary Medicine. 170:104713. https://doi.org/10.1016/j.prevetmed.2019.104713
SANDAM NP, Prakash D, Thimmareddy P. 2021. Immunocontraceptive potential of a GnRH receptor-based fusion recombinant protein. Journal, genetic engineering & biotechnology. 19(1):63. https://doi.org/10.1186/s43141-021-00164-9
SERRANO H, Pérez-Rivero JJ, Martínez-Maya JJ, Aguilar-Setién A, Pérez-Martinez M, García-Suárez MD. 2008. Fluorescence and inmunohistological detection of estrogen receptors in dog testis and epidydimis after oral coumestrol administration. Neuroendocrinology Letters. 29(6):977–980. https://doi.org/10.1007/s11259-008-9077-3
SMITH LM, Hartmann S, Munteanu AM, Dalla Villa P, Quinnell RJ, Collins LM. 2019. The Effectiveness of Dog Population Management: A Systematic Review. Animals (Basel). 9(12):1020. https://doi.org/10.3390/ani9121020
WANG D, Xie J, Zhu X, Li J, Zhao D, Zhao M. 2014. A recombinant estrogen receptor fragment-based homogenous fluorescent assay for rapid detection of estrogens. Biosensors and Bioelectronics. 55:391-395. https://doi.org/10.1016/j.bios.2013.12.050
WYSE JM, Latif S, Gurusinghe S, Berntsen ED, Weston LA, Stephen CP. 2021. Characterization of Phytoestrogens in Medicago sativa L. and Grazing Beef Cattle. Metabolites.11(8): 550. https://doi.org/10.3390/metabo11080550s
ZUVELA E, Matson P. 2020. Performance of four chambers to measure sperm concentration: results from an external quality assurance programme. Reproductive biomedicine online. 41(4):671-678. https://doi.org/10.1016/j.rbmo.2020.07.008